


 |
|
Glutamate (Glu) is the most excitatory neurotransmitter in the brain of all vertebrates and many advanced invertebrates, where it mediates critical functions in development, normal physiology, and complex behavior. However, several factors make Glu an unlikely signaling molecule. Most importantly, though Glu is made in large amounts by all cells, the handling of Glu is in animals’ brain is very dangerous, because excessive Glu signaling is highly toxic to many neurons. Therefore, in most of the evolutionary tree, animals do not adopt Glu as their neurotransmitter of choice till later in evolution, when they acquire very effective protective barriers that insolate the synapses. Surprisingly, nematodes like C. elegans are a clear exception to this rule. They juggle Glu around more than 50% of their neurons, without the presence of traditional protective barriers such as isolation of synapses by glia. Hoping to learn from this exception more about the rule, we ask how do nematodes do it?
C. elegans, like many other animals, use Glu Transporters (GluTs) to clear excess Glutamate from synaptic connections. Indeed, our previous work showed that similarly to mammalian GluTs, some of the nematode’s GluTs (e.g., GLT-1 & GLT-4) are located close to the synapses in the animal’s “brain” – the nerve-ring. The nerve-ring GluTs also seem to be very similar to mammalian GluTs in their molecular structure. However, many other worm GluTs (GLT-3, GLT-6, and GLT-7) are expressed on an elongated tubular cell called the canal cell. From this unusual location (which is some distance away from the nerve-ring synapses), the canal-cell GluTs exert powerful control over synaptic activity in the nerve-ring. Adding to the mystery of the function of the C. elegans canal-cell GluTs are some subtle but very significant modifications seen in their molecular structure, not seen in any GluT in any other organism (except other nematodes).
We use a microfluidic chip
to trap intact animals and stimulate specific circuits. We use
transgenic expression of the Ca2+ -sensitive fluorescence reporter
GCaMP, expressed under specific promoters, to record postsynaptic
responses to these stimuli. We determine the effect of knockout of
different GluTs on the activity of these circuits, and follow changes
such as spillover between circuits to determine the physiology of
glutamate clearance and circuit isolation in the absence of anatomical
separation between circuits.
We suggest that these unique molecular
properties hint that canal cell GluTs might have a special
physiological role. We are asking what are the functional
consequences of these modifications. Do these unusual GluTs provide
the basis to the amazing ability of nematodes to handle as a risky
transmitter as Glutamate? 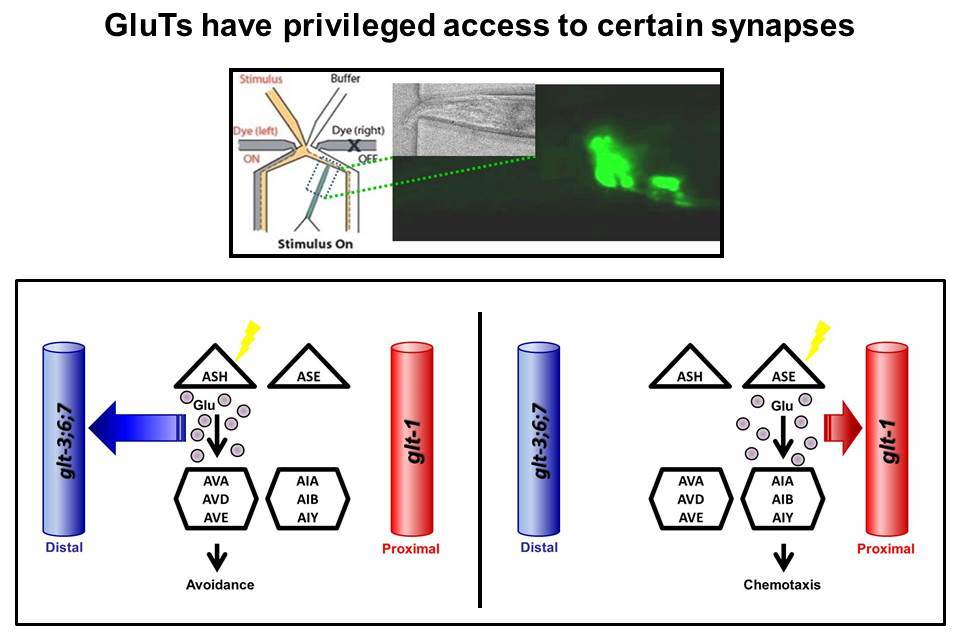 |
Physiol. Pharm. & Neurosci, City College, C. elegans@CUNY

