 |
|
A Script for a Requiem - The Mechanism of Excitotoxicity
In stroke and a range of chronic
neurodegenerative diseases, neurodegeneration results from the
accumulation and exaggerated action of Glutamate (Glu). In these
conditions, Glu accumulates in synapses because Glu Transporters
(GluTs) can not
We take advantage of the powerful tools available in C. elegans
research to perform a genetic screen that is unbiased by previous
understanding. We have recently produced a unique and reliable C. elegans model
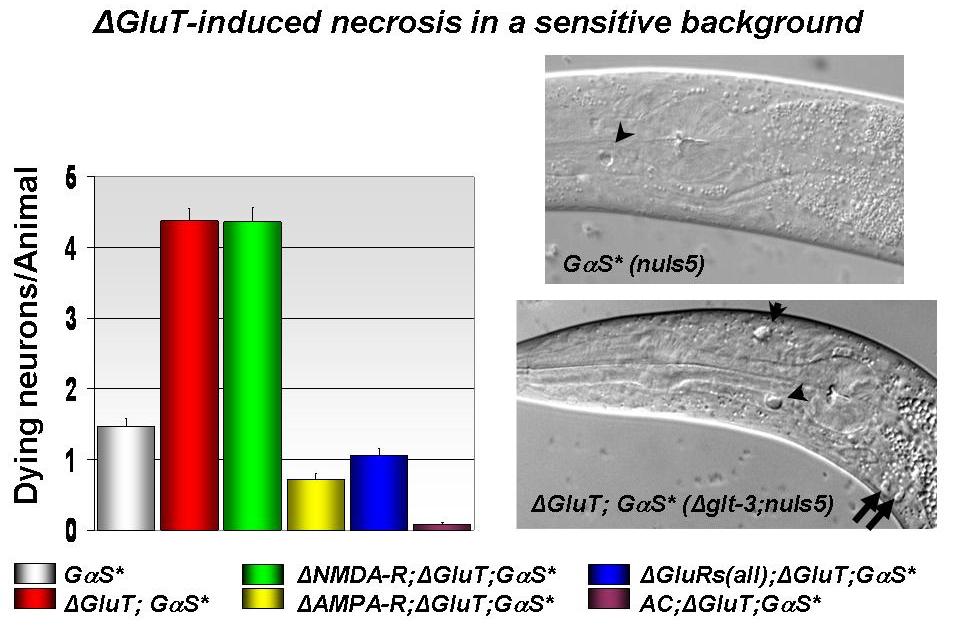
for excitotoxic neurodegeneration by knocking out GluTs in a sensitized
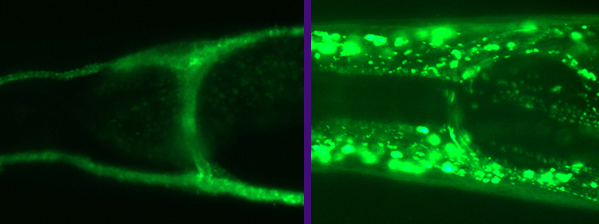
background, resulting in necrosis of neurons postsynaptic to
glutamatergic connections. The excitotoxic necrosis depends of the Ca2+
-permeable AMPA receptors expressed in these synapses. We now screen
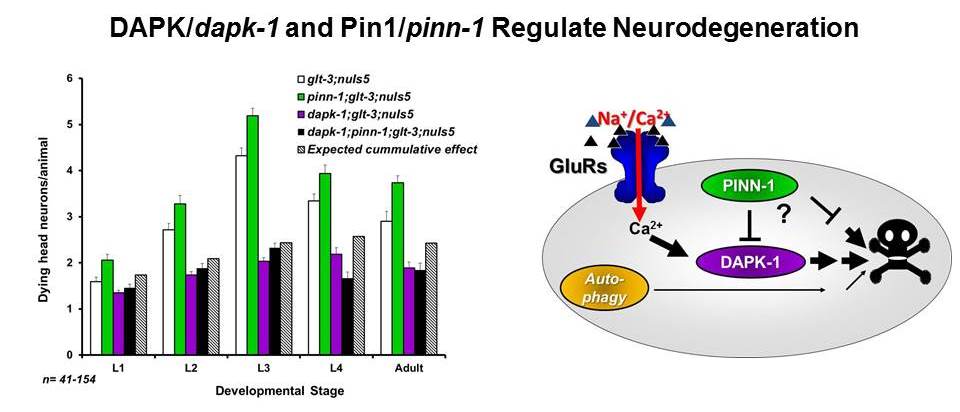
for genes that are specifically involved in neurodegeneration by
methodically looking for interruptions in other genes that can block,
reduce or enhance excitotoxic neurodegeneration.
This project is uniquely poised to
provide a whole-organism view of Glu balance and to pin-down new
genetic means to stop/slow excitotoxicity, without the bias of prior
dogmas, addressing a major health concern of the general population
with a fresh and promising approach.
|
Physiol. Pharm. & Neurosci, City College, C. elegans @ CUNY
 function when brain cells do not have enough energy.
The overstimulation of post synaptic Glu receptors triggers a
neurodegenerative cascade called excitotoxicity. Currently there is
no therapy for excitotoxicity, and a large group of recent clinical
trials that were based on our current understanding of excitotoxicity
ended with disappointment. These failures indicate that in spite of
the urge to go directly to clinical and translational research, public
health might be better served in the long run if we expand the basis
of our understanding of the cellular pathways that lead to
excitotoxic neurodegeneration.
function when brain cells do not have enough energy.
The overstimulation of post synaptic Glu receptors triggers a
neurodegenerative cascade called excitotoxicity. Currently there is
no therapy for excitotoxicity, and a large group of recent clinical
trials that were based on our current understanding of excitotoxicity
ended with disappointment. These failures indicate that in spite of
the urge to go directly to clinical and translational research, public
health might be better served in the long run if we expand the basis
of our understanding of the cellular pathways that lead to
excitotoxic neurodegeneration.