Defying the Odds:
Cell Stress Resistance, CREB, and Neuroprotection in Excitotoxicity
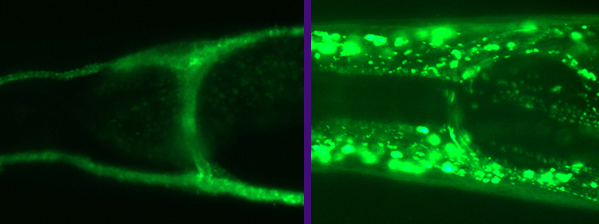
 Stroke / brain ischemia is caused by toxic accumulation of Glutamate
(Glu), leading to excitotoxic neurodegeneration. Glu is toxic to many
brain cells throughout development and aging. However, researchers
observed an increased susceptibility, where aged neurons are far more
likely than young neurons to die following Glu insult. Currently we
know very little about the workings of the internal clock that
triggers a concerted decline of many body systems during aging. We do
not know why older neurons seem to be more fragile and susceptible to
excitotoxicity.
Stroke / brain ischemia is caused by toxic accumulation of Glutamate
(Glu), leading to excitotoxic neurodegeneration. Glu is toxic to many
brain cells throughout development and aging. However, researchers
observed an increased susceptibility, where aged neurons are far more
likely than young neurons to die following Glu insult. Currently we
know very little about the workings of the internal clock that
triggers a concerted decline of many body systems during aging. We do
not know why older neurons seem to be more fragile and susceptible to
excitotoxicity.
Exciting
new research of an evolutionary-conserved mechanism of cellular aging
and stress resistance is currently emerging. Age-defying mutations in
the Insulin/IGF-1 Signaling (IIS) cascade identified in worms were also
shown to protect them from cell stress and toxic proteins by activating
a cell-protective transcriptional program regulated by the
transcription factor FoxO/DAF-16. These effects are also seen in
mammals, since the cellular-aging/stress resistance signaling pathways
are highly conserved from worms to humans.
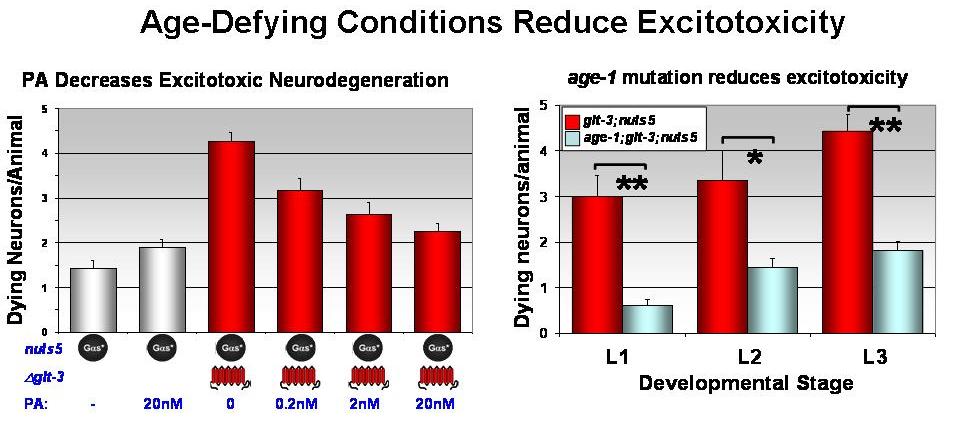
We ask if the increased cell resistance seen in age-defying mutant
nematodes also protects them from Glu-accumulation-triggered
excitotoxic neurodegeneration. The availability of particularly
powerful research tools in C. elegans,
the rich repertoire of aging-affecting mutations, and the advanced
understanding of aging signaling pathways in the worm, together with
our recent demonstration of excitotoxicity model in this system, make C. elegans the
system of choice to study the connection between aging and
susceptibility to excitotoxic neurodegeneration. We use pharmacological
and genetic analysis to look for processes that affect cellular
aging/stress resistance and the susceptibility to excitotoxicity.
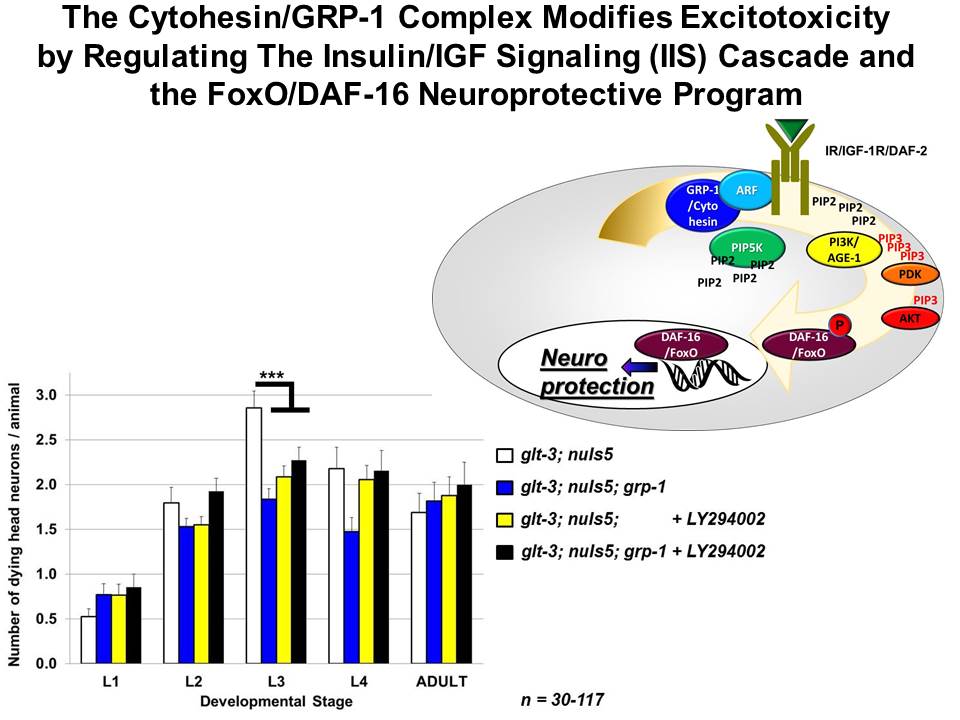
We have recently found that the Cytohesin/GRP-1 complex regulates the
IIS cascade to control the neuroprotective activity of FoxO/DAF-16 in
excitotoxic necrosis. We also found that another classic transcription
factor, CREB, also controls neuroprotection. We are currently studying
how the signaling cascades that control there two transcription factors
might cooperate to provide specificity in neuroprotection from
excitotoxic necrosis.
|
|